NOn-VIral gene modified STEM cell therapy
Start
November 2022
Duration
48 months
Budget
€3 644 418
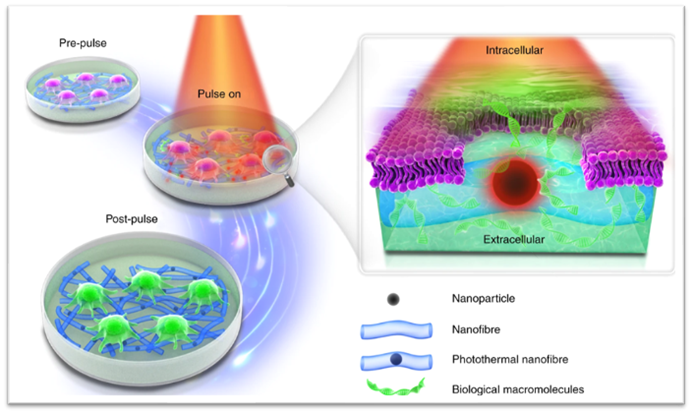
Photoporation
Cell membrane permeabilization with photothermal nanofibers is a clinically applicable next-generation transfection technology holding great promise for safer and more efficient engineering of therapeutic cells. Currently, the production of cell therapy products involves a long and costly process notably due to the use of viral vectors, which hampers the accessibility of the therapy to patients. To overcome these difficulties, physical transfection methods recently emerged as promising opportunities.
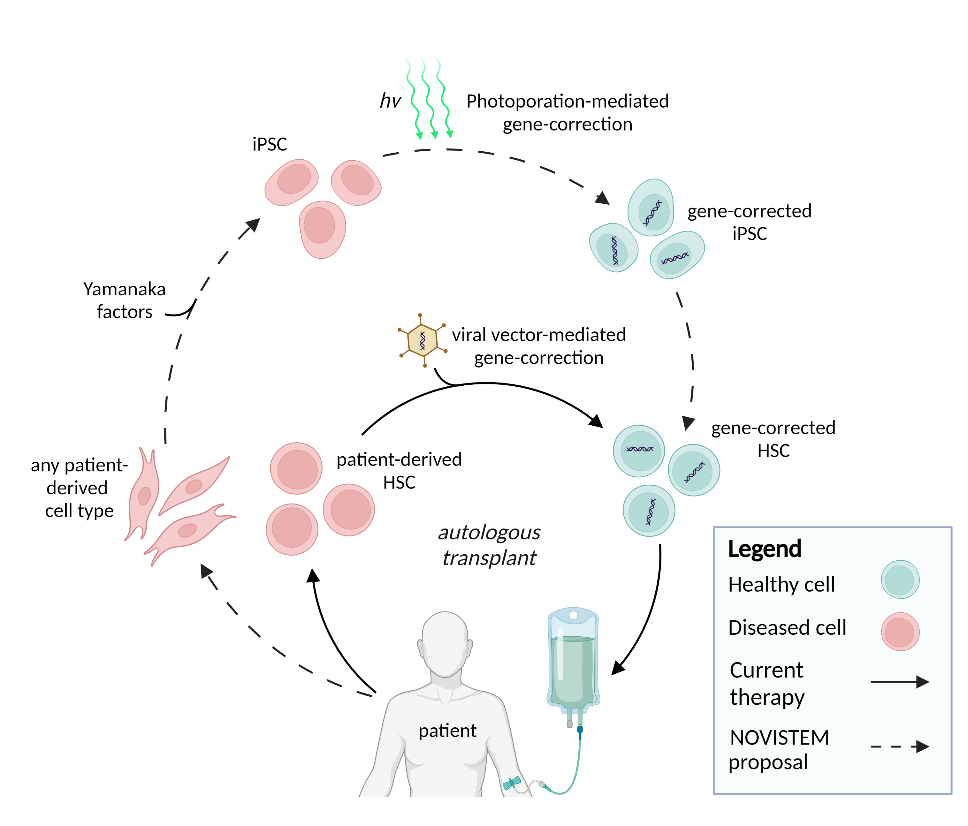
Hematopoietic Stem Cells
To cure genetic hematological disorders, patient-derived hematopoietic stem cells (HSCs) can be gene-corrected and transplanted. Yet, the limitations of editing and expanding primary HSCs in vitro, along with risks linked with viral vectors for gene correction, impede this therapy’s implementation. To overcome these challenges, we propose to genetically correct patient-derived iPSCs through photoporation and subsequently differentiate these into transplantable HSCs by recapitulating the developmental environment.
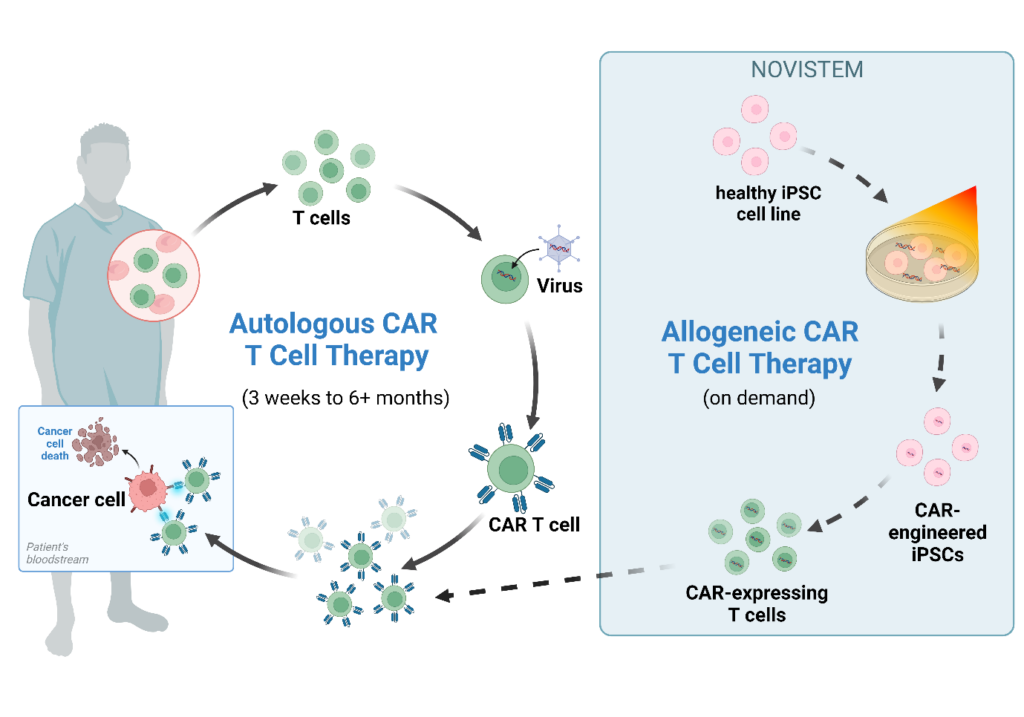
Universal CAR T therapy
In addition to the current highly individualized CAR T therapy and the inept decentralized production model, the subpar quality of patient-isolated immune cells limit the implementation of a potent universal CAR T cell therapy. With the use of healthy donor induced pluripotent stem cells (iPSCs) as starting material, combined with an expertise in T cell biology, the generated CAR T phenotype can be enhanced to be more effective for anti-cancer therapy. After optimisation of the protocols and with the use of CAR-iPSC storage banks, the therapy can be scaled up to increase the target patient population.