Our Vision
The core of this project are induced pluripotent stem cells (iPSC), which are derived from somatic cells and reprogrammed to generate a blank slate, allowing us to manipulate their development into potent cells of interest. Even though they are highly considered as a medical revolution and an unlimited source of any type of human cell, wide application of iPSCs for therapeutic purposes has been hampered by various challenges. Funded by the European Innovation Council, the NOVISTEM project aims to streamline the genetic modification of iPSCs and their subsequent use in manufacturing cell therapy products. With the use of an innovative gene delivery system, called photoporation, the engineering of these cells will be optimized. Moreover, we will employ these iPSCs to generate hematopoietic stem cells capable of regenerating the deficit blood cell lineages in known blood disorders. Finally, these iPSCs will also be engineered to encode chimeric antigen receptor (CAR) and induced to differentiate to CAR-expressing T cells by targeting a signalling pathway that drives T cell development. This will allow us to produce large numbers of well-characterised CAR-T cells for clinical use.
Why?
Despite the increasing awareness that cell and gene-therapy approaches have tremendous biomedical potential, their broad clinical application has been challenging due to prolonged and expensive production times and the emergence of severe immune- and gene-delivery dependent side effects. In this project, we aim to establish a stream-lined and high-throughput protocol for iPSC-based cell therapy by combining a novel technological platform for gene delivery with a breakthrough biological concept that will permit to manufacture functional, gene-corrected blood forming stem cells and CAR T cells.
How?
To achieve this, we will use and optimize photoporation as a non-viral gene delivery method for CRISPR-mediated and site-specific gene-editing to obtain controlled CAR expression and for performing gene-correction in iPSCs. From these gene-modified iPSCs, we will generate CAR T cells and blood forming stem cells, respectively, by selectively targeting a signaling pathway that we established to be critical in human blood cell development and particularly T cell development. Following functional validation of the generated cell products, we will optimize the current protocols to increase the potential for clinical implementation and establish a high-throughput photoporation platform to generate a large number of CAR expressing iPSC lines from different ages, sex and ethnicities to demonstrate the population-wide implementation potential of our approach. This will allow to generate a bank of well-characterized, HLA-defined CAR expressing iPSC that can be used as of-the-shelf cell therapy products, thereby significantly advancing the currently implemented adaptive CAR T cell approaches by reducing the production costs and time, by selectively targeting the CAR into a well-controlled location which will prevent variability and by facilitating the production and evaluation of novel CARs for other cancer entities such as solid tumours.
Photoporation
When used for therapy, cells are often modified through the introduction of exogenous molecules in their cytosol, a process called transfection. While a wide variety of transfection technologies have been developed, viral vectors currently hold a quasi-monopoly when it comes to therapeutic cell production. However, the use of viral vectors also comes with safety, productivity and financial limitations hampering the mass distribution of innovative and highly potent cell therapy products. As an alternative to viral vectors, physical transfection technologies, with electroporation at their forefront, emerged as promising alternatives enabling high throughput cell transfection. However, physical transfection, and electroporation in particular, typically show a highly cytotoxic profile decreasing the efficiency of the manufacturing procedure, affecting the therapeutic potency of the final cell product, and therefore limiting their use in the clinical setting.
After more than one decade of research and development on a novel physical transfection modality termed photoporation, the Braeckmans lab (Ghent University, Belgium) recently developed photothermal electrospun nanofibers (PEN) enabling harnessing the full potential of photoporation for therapeutic cell engineering. This novel technology exploits the interaction of nanosentisizers -embedded within a nanofiber substrate- with a laser light to gently induce pores in the cell membrane, thereby efficiently enabling transfection. In comparison to the state-of-the-art technologies, PEN photoporation simultaneously solves the safety and productivity limitations associated with viral vectors, and the toxicity limitations associated with electroporation.
Whereas PEN photoporation comes with important advantages making it amenable to therapeutic cell manufacturing, this technology has not been evaluated yet for the delivery of nucleic acids (mRNA and DNA) to stem cells. Optimizing photoporation for minimally perturbative delivery of large nucleic acids to e.g. iPSCs would, however, simultaneously open a myriad of opportunities to produce high-quality cell therapy products retaining their full therapeutic potency while enhancing cost-effectiveness relative to the currently available cell therapies. Consequently, further developing the PEN photoporation platform for cellular transfection with a wide array of effector molecules is one of the core objectives of the NOVISTEM project.
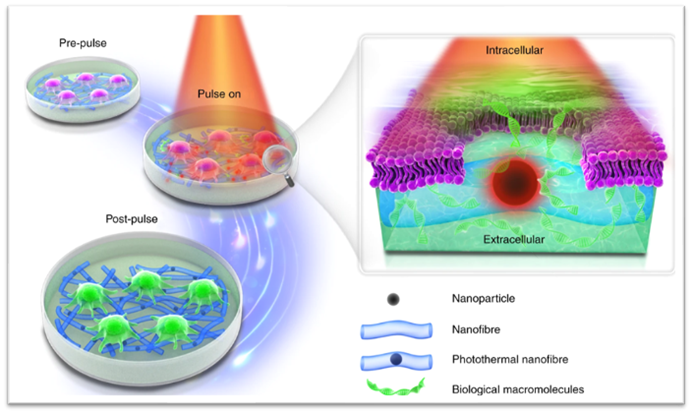
Originally published in Xiong et al., Photothermal nanofibers enable safe engineering of therapeutic cells, Nature Nanotechnology, 2021.
Hematopoietic stem cells
Throughout life, blood cells are constantly being replenished by a process known as hematopoiesis, whereby hematopoietic stem cells go through continuous self-renewal and differentiation. To ensure the production of various blood cell types, such as red blood cells, T-cells or B-cells, specific signals are needed to guide the stem cells along the correct developmental pathways. Sometimes, genetic errors or mutations occur in these hematopoietic stem cells, which can lead to a range of hematological and immune disorders, such as leukemia, sickle cell anemia, thalassemia major and severe combined immunodeficiency.
Transplanting healthy hematopoietic stem cells isolated from donors remains the main curative treatment for such genetic blood disorders, as these healthy cells are capable of restoring the entire blood system of the patient. In Europe alone, over 40,000 transplantations were performed in 2014. However, the patient’s and donor’s human leukocyte antigen (HLA)-types need to match to ensure success. If there is a significant mismatch, there can be severe complications such as immune rejection, infection and graft-versus-host disease. This means that finding a suitable donor can be difficult, so that patients may wait a long time for live-saving treatment. As an alternative, a patient’s own hematopoietic stem cells can also be used for transplantation. For example, in leukemia patients, healthy stem cells can be isolated and transplanted, but this carries the risk of contamination with malignant leukemic cells and a relapse of the disease can occur upon transplantation. In the case of hereditary genetic diseases, where all of the patient’s cells carry the disease mutation, the hematopoietic stem cells would need to be genetically corrected prior to transplantation which is currently difficult to do. Another challenge is the limited amount of hematopoietic stem cells that can be isolated from any donor or patient, as a minimum of 2 million CD34+ stem cells per kg body weight of the patient is required to successfully restore the hematopoietic system.
The invention of induced pluripotent stem cells (iPSC) was seen as a revolution in the field of regenerative medicine. These cells are made in the lab by reprogramming any cell (e.g. skin cells, blood cells…) into a pluripotent state, meaning that they can differentiate into a desired type of cell. Similarly, the possibility of deriving an unlimited supply of healthy, hematopoietic stem cells from patient-specific iPSCs holds unique potential to solve current problems associated with stem cell transplantation. However, to date, no method has successfully produced bona fide hematopoietic stem cells from induced pluripotent stem cells. By recapitulating signals that occur during embryonic development, we aim to create a method that allows us to generate bona fide hematopoietic stem cells from healthy or gene-corrected induced pluripotent stem cells.
CAR T cell therapy
CAR T cell therapy is a cancer immunotherapy where T cells are isolated from the peripheral blood of patients and modified via the introduction of a CAR, a Chimeric Antigen Receptor. This aides the T cells to better recognize and kill their target cancer cells and thus optimizes the anti-cancer defence.
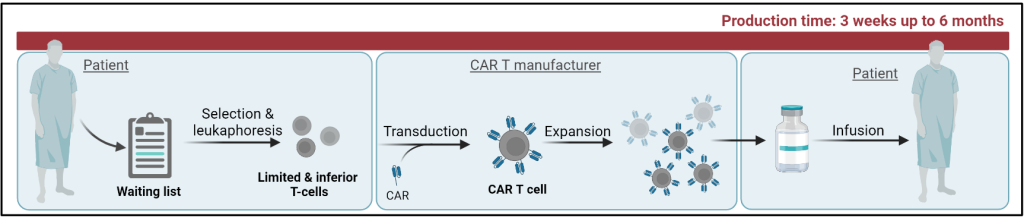
The next step within the CAR T field is the development of a universal off-the shelf CAR T cell therapy that is applicable to a broader range of patients and overcomes the drawbacks associated with the standard approach; prolonged production, high costs and limited access. The need for a more high-throughput and faster production system prompts the use of induced pluripotent stem cells (iPSC) as the starting material to facilitate product storage into iPSC banks and the generation of more potent T cell products. As opposed to the standard approach, where one patient only accommodates for the therapy of the same patient; the use of universal donor samples potentiates the treatment of multiple patients at once. The subpar quality of T cells isolated from patients denotes an inferior starting material for therapy production, whereas the controlled differentiation of iPSCs towards T cells of interest promises a potential strategy of generating higher quality T cell products.
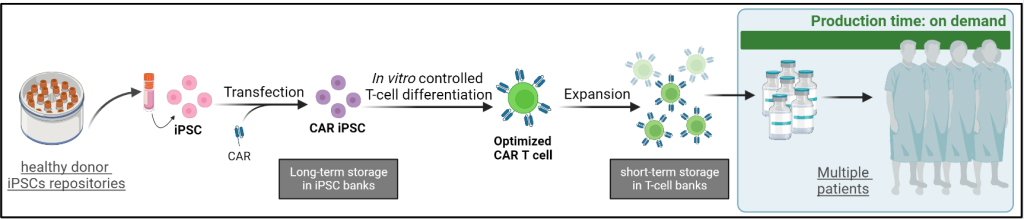
The current universal CAR T cells are limited in their T cell biology and the in vitro generation of T cells from iPSCs requires optimization to generate adequate and effective T cells. The use of photoporation, a more gentle mechanism to transfect the iPSCs with the CAR, combined with an innovative T cell differentiation system, the CAR T cell products would be of higher quality to implement as a universal off-the-shelf CAR T therapy.